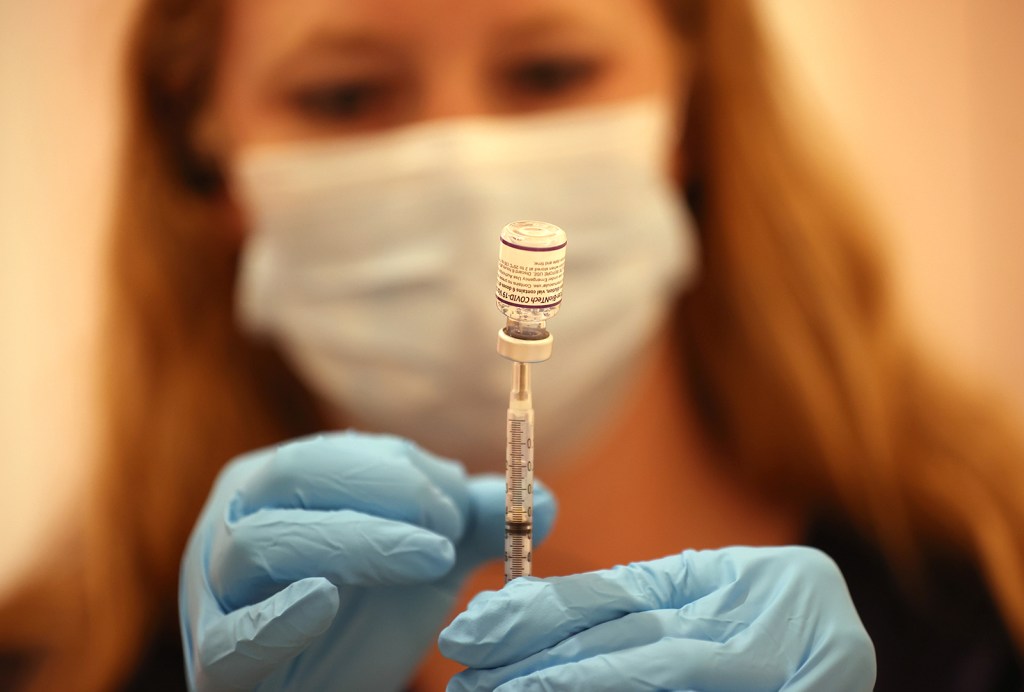
Ahead of the fall respiratory virus season, the Food and Drug Administration has approved two updated COVID-19 vaccines. The new messenger RNA, or mRNA, vaccines from Moderna and Pfizer/BioNTech are formulated to better target variants that are currently circulating and will replace outdated vaccines.
“The new vaccine that was just approved by the FDA is essentially a COVID vaccine targeting a different strain of the COVID virus than was in the original vaccine or in the bivalent vaccines that came out last year. It’s still a COVID vaccine, but it’s now targeting the XBB.1.5 strain, which has been the omicron-type virus that’s been circulating throughout the U.S. and most parts of the world since the beginning of this year,” says Dr. Priya Sampathkumar, a Mayo Clinic infectious diseases expert.
Is it considered a booster?
“It’s not exactly a booster. I would liken it to the updated influenza vaccine that comes out each year. The influenza vaccine is updated each year as the strains that they protect against change year from year,” says Dr. Sampathkumar.
Who should receive the 2023-2024 COVID-19 vaccine?
The Centers for Disease Control and Prevention panel of vaccine experts voted to recommend the new 2023-2024 COVID-19 vaccine to all Americans 6 months of age and older.
“The COVID vaccine definitely should be taken by those at highest risk of complications from COVID, and that includes older people, people with weakened immune systems, very young children. These are the people that we are seeing have significant complications from COVID,” says Dr. Sampathkumar.
Health experts also are urging people to get vaccinated for influenza. Experts say it is safe to get both the COVID-19 and flu vaccines at the same time. Both vaccines have been shown to prevent the most serious complications of COVID-19 and the flu, which include hospitalization and death.
“The (flu) vaccine is recommended for…
Read the full article here