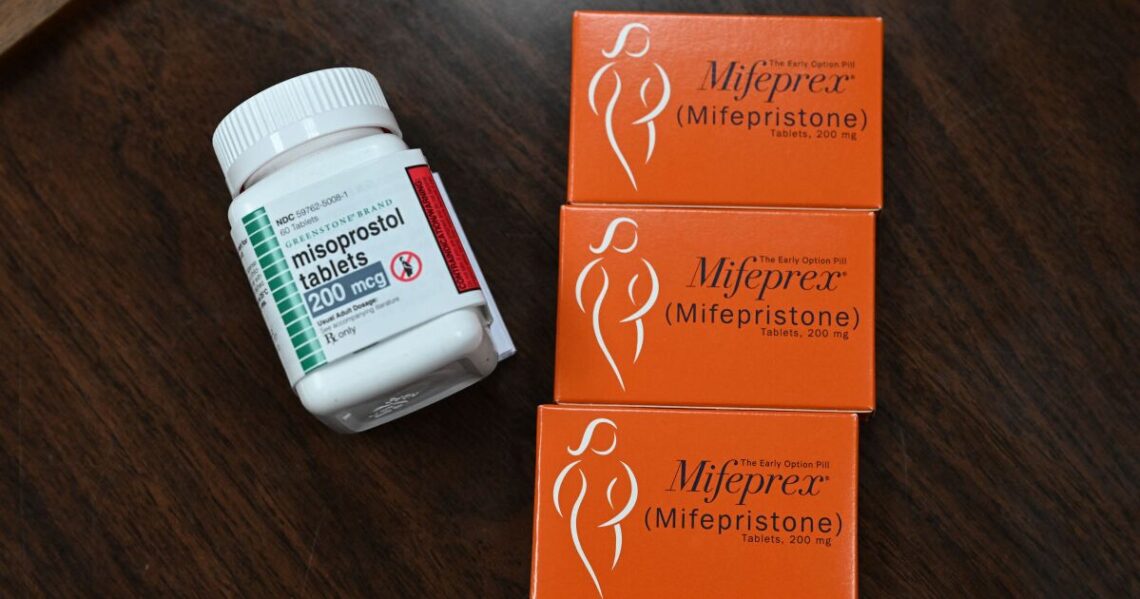
On April 7, a federal court judge in Texas ordered that the drug mifiprisone be pulled off the market. Judge Matthew Kacsmaryk ruled the Food and Drug Administration had violated federal rules that allowed for the accelerated approval of some drugs and erred in its scientific assessment of the abortion pill.
Mifipristone is an oral medication used with misoprostol to induce abortion or to manage a miscarriage. That protocol was approved by the FDA in 2000. When taken together, they end a pregnancy without the need for surgery in 99% of cases.
Another federal court judge in the state of Washington issued a conflicting ruling, directing the Food and Drug Administration not to alter its approval.
Because these two federal court rulings conflict, the U.S. Supreme Court may resolve this conflict and decide the merits of both cases.
The decision has nationwide ramifications for millions of women and the future of drug approvals.
What is the timeline?
Supreme Court Justice Samuel Alito placed a hold on Friday on a lower court ruling that restricts access to the abortion drug mifepristone until Wednesday night.
Alito also instructed that any responses be filed by April 18 at noon. Alito’s order keeps the status quo in place, and mifipristone legal and available nationwide.
Friday’s hold was in response to a formal request earlier in the day from the Justice Department to block a federal appeals court decision that limits access to the drug.
Portions of a Texas district court’s order that limits mifepristone would have otherwise taken effect April 15, but Alito’s order put it on pause.
Mifipristone remains available
Despite all the headlines, technically nothing has changed.
“There has been no change yet, in the law or the regulations of the Food and Drug…
Read the full article here