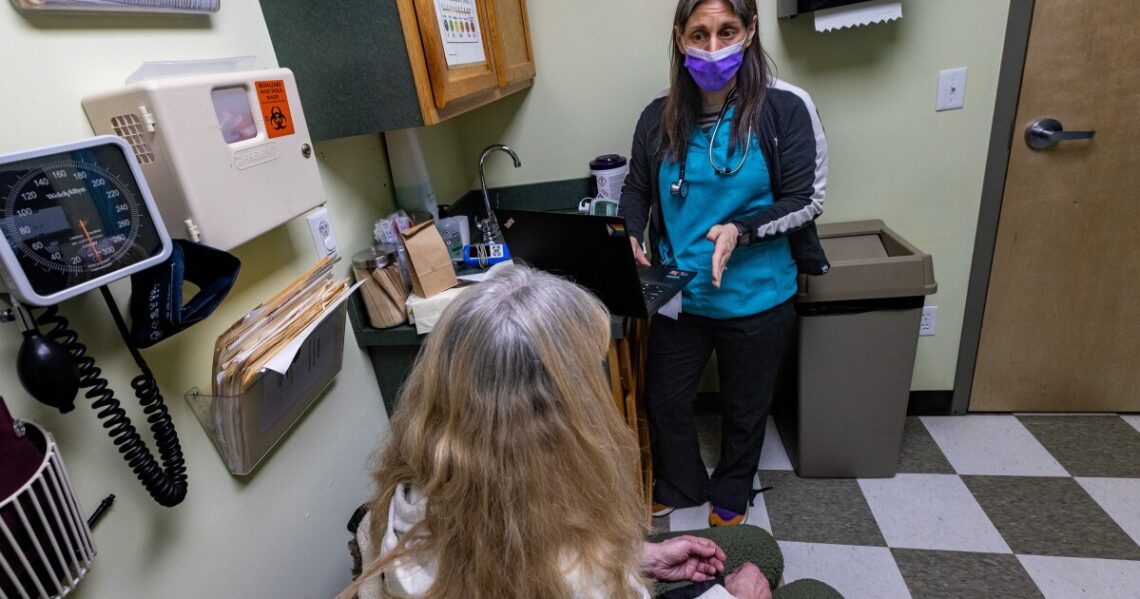
For two decades — as opioid overdose deaths rose steadily — the federal government limited access to buprenorphine, a medication that addiction experts consider the gold-standard for treating patients with an opioid use disorder. Study after study shows it helps people continue addiction treatment while reducing the risk of overdose, and death.
Clinicians who wanted to prescribe the medicine had to complete an 8-hour training. They could only treat a limited number of patients and had to keep special records. They were given a Drug Enforcement Administration (DEA) registration number starting with X, a designation that many doctors say made them a target for drug enforcement audits.
“Just the process associated with taking care of our patients with a substance use disorder made us feel like, ‘boy, this is dangerous stuff,'” says Dr. Bobby Mukkamala, who chairs the American Medical Association’s task force on substance use disorder.
“The science doesn’t support that but the rigamarole suggested that.”
That rigamarole is mostly gone. Congress eliminated what became known as the “X-waiver” in legislation President Biden signed late last year. Now begins what some addiction experts are calling a truth serum moment.
Was the X-waiver and the burdens that came with it the real reason only about 7% of clinicians in the U.S. were cleared to prescribe buprenorphine? Or was it an excuse that masked hesitation about treating addiction, if not outright disdain for these patients?
There’s great optimism among some leaders that getting rid of the X-waiver will expand access to buprenorphine and reduce overdoses. One study from 2021 shows taking…
Read the full article here