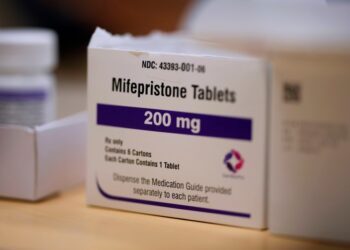
The U.S. Food and Drug Administration late last year approved two breakthrough gene therapies for sickle cell disease patients. Now a new federal program seeks to make these life-changing treatments available to patients with low incomes — and it could be a model to help states pay for other expensive therapies.
The new sickle cell treatments have brought hope to those with the debilitating blood disorder, which is hereditary and disproportionately affects Black people. But the therapies come with a price tag of as much as $3 million for a course of treatment, which can take up to a year. Despite those high upfront costs, cell and gene therapies have the potential to reduce health care spending over time by addressing the underlying cause of the disease.
Under the so-called Cell and Gene Therapy Access Model, the federal government will negotiate discounts with sickle cell drug manufacturers Vertex Pharmaceuticals, CRISPR Therapeutics and Bluebird Bio on behalf of state Medicaid agencies, which provide health care coverage to low-income patients. To participate, state Medicaid agencies must agree to prices based on those negotiations, and pledge to provide broad access to the therapies.
The federal government said it will negotiate an “outcomes-based agreement” with the companies, meaning the prices for treatments will be tied to whether the therapy improves health outcomes.
If there are no in-state treatment centers, Medicaid agencies would pay for patients to receive the therapies in another state. Between 50% and 60% of sickle cell patients are on Medicaid, according to federal estimates.
The federal Centers for Medicare & Medicaid Services (CMS) launched the program, which is scheduled to begin next year, in response to President Joe Biden’s 2022 executive order on lowering prescription drug prices.
CMS officials say the framework is being tested with sickle cell disease treatments first, but that other…
Read the full article here