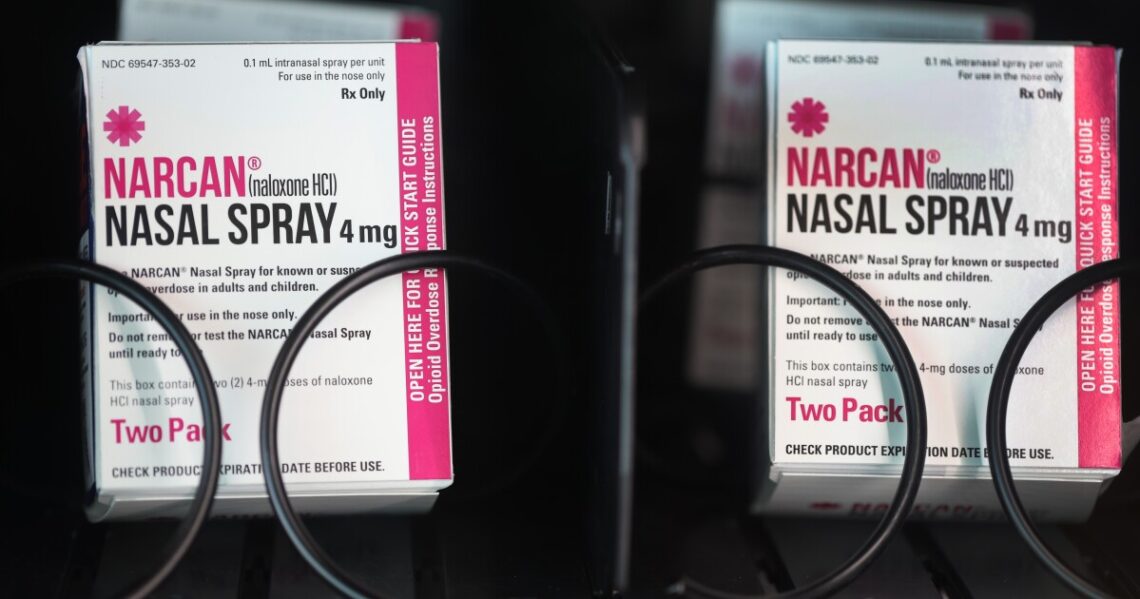
The overdose-reversing drug Narcan could soon be available to buy over the counter without a prescription, the Food and Drug Administration announced Wednesday.
The FDA’s approval of the nasal spray Narcan — the brand name for the drug naloxone — means the medication could be more widely available across the U.S. as the country continues to grapple with an opioid epidemic.
“Today’s action paves the way for the life-saving medication to reverse an opioid overdose to be sold directly to consumers in places like drug stores, convenience stores, grocery stores and gas stations, as well as online,” the FDA said in a statement.
Emergent BioSolutions, the drug company that produces Narcan, said on Wednesday that it hoped to make the nasal spray available on store shelves and at online retailers by late summer. It did not immediately say how much it would cost.
“Today’s landmark FDA OTC approval for Narcan Nasal Spray marks a historic milestone as we have delivered on our commitment to make this important emergency treatment widely accessible, given the alarming rates of opioid overdoses occurring across the country,” Emergent BioSolutions CEO Robert G. Kramer said in a statement.
Some states and harm-reduction groups have offered naloxone for free to residents. But typically those who wanted to buy Narcan had to obtain it at a pharmacy with a prescription.
Public health experts, harm-reduction advocates and many politicians have argued that those barriers meant fewer people would get the life-saving medication they needed in time.
The FDA approval comes as the U.S. continues to see a staggering number of opioid-related deaths, driven in large part by the spread of synthetic opioids such as illicit fentanyl.
According to data from the…
Read the full article here